Department of Genetics and Molecular Oncology
Innovative Medical Forum – Department of Genetics and Molecular Oncology (ZGiOM) presents:
- modern infrastructure,
- world-class level technologies,
- a team that systematically improves qualifications in Poland and abroad.
This contributes to the success in implementing new services in line with global standards of genetic cancer diagnostics.
In 2014, when the Department of Genetics and Molecular Oncology was established, testing was carried out using two methods, i.e. real-time PCR and in situ hybridization. Only a few molecular biomarkers (3-4 genes) were analysed with the use these methods. Due to the huge boom in oncogenetics, there has been a significant expansion of diagnostic procedures with new, innovative technologies: Next Generation Sequencing (NGS), capillary sequencing using the Sanger method, automated process of fluorescence in situ hybridization. The number of tested biomarkers has also increased – currently at ZGiOM more than 25 biomarkers are assessed with the use of the abovementioned methods.
MODERN INFRASTRUCTURE
The Department of Genetics and Medical Oncology is located in the Innovative Medical Forum (IFM). It is a unique place, a pioneering investment, the project of which from the very beginning was based on modern medical, diagnostic and educational infrastructure. IFM creates a bridge between diagnosticians – geneticists, the clinic and the patient. Its task is to integrate science with the clinic by presenting scientific achievements in translational medicine, in particular the application of molecular biology in the field of genetic diagnosis of cancer.
WORLD-CLASS LEVEL TECHNOLOGIES
The Department of Genetics and Molecular Oncology stands out from other centres in the region thanks to its innovative equipment, i.e.:
- using next-generation NGS sequencing technology – MiniSeq sequencer and NextSeq550 high-throughput sequencing system.
- the latest SeqStudio Genetic Analyzer capillary sequencing system – as the first one installed in Poland for DNA sequencing using the Sanger method,
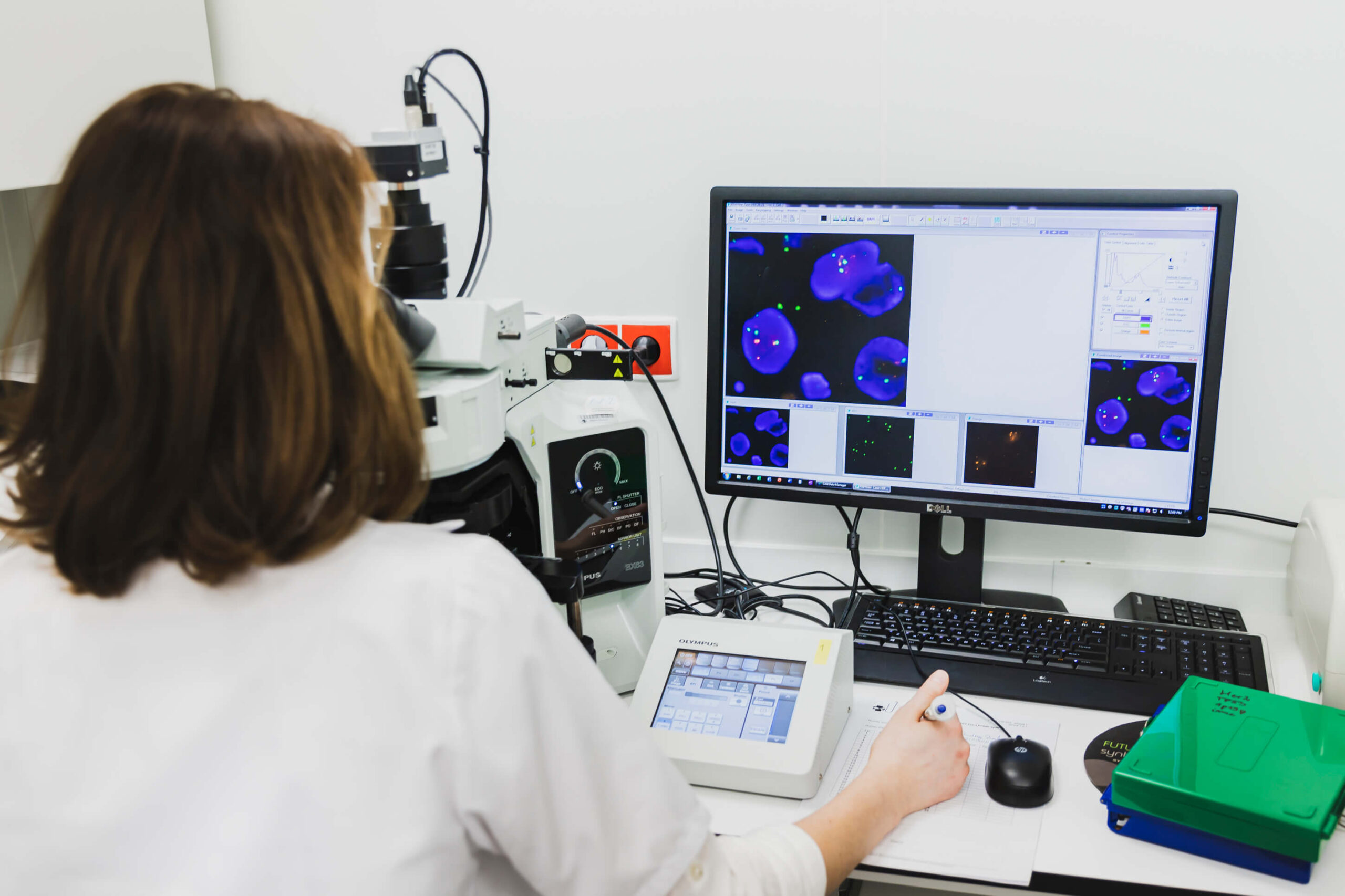
- Discovery Ultra apparatus, which automated the process of fluorescence in situ hybridization and the Olympus fluorescence microscope with modern Applied Spectral Imaging (ASI) software for automatic cytogenetic evaluation in solid tumours.
STAFF OF DEPARTMENT OF GENETICS AND MOLECULAR ONCOLOGY
It is not only about equipment and modern technologies – the success of ZGiOM is primarily determined by the knowledge and competence of the interdisciplinary team and reliable work. The wealth of ZGiOM is the medically (diagnostically) and scientifically developing staff comprising diagnosticians, doctors and doctoral students. The research team led by Associate Professor Marzena Anna Lewandowska, MD, PhD, MBA systematically raises its qualifications through participation in trainings in Poland and abroad (in Germany, Belgium, Korea and the USA). Scientific work under the direction of professor Lewandowska is published in international scientific journals, doctoral dissertations have significant substantive value awarded with distinction (K Kamińska, MD, PhD, M Kubiak, MD, PhD), scholarship of the Minister of Science and Higher Education for achievements in science.

GENETIC DIAGNOSTICS IN ACCORDANCE WITH THE WORLD STANDARDS
The equipment of the Department of Genetics and Molecular Oncology and a competent team of professionals allow for the implementation and provision of medical services in line with current world standards. ZGiOM is a precursor of genetic diagnostics of neoplastic tissue in the region and neighbouring voivodships. In response to the current needs, resulting from the latest achievements in the field of personalized medicine, we offer cancer patients the analysis of approximately 25 prognostic and predictive biomarkers. Genetic testing supports the therapeutic decisions of the doctors of the Oncology Centre.
We provide the necessary genetic information to qualify patients for targeted therapy: breast cancer, ovarian cancer, non-small cell lung cancer, colon cancer, malignant melanoma, gastrointestinal stromal tumours (GIST), as well as genetic tests of glioblastoma, B-cell neoplasms, Ewing’s sarcoma or synovial sarcoma.
The use of the innovative NGS technology allows to significantly expand the group of oncological patients who may be offered personalized treatment. The ability to analyse mutations in the BRCA1/2 gene by Next Generation Sequencing method meets the needs of oncologists in the scope of qualifying patients with ovarian cancer to the drug program with the use of a targeted drug, as well as surgeons and clinical geneticists in the scope of the algorithm of management of patients with breast cancer.
Research procedures at ZGiOM performed in 2014-2018 in the field of cancer diagnostics were focused on the analysis of genetic changes in cancer tissue. In 2019, the Department of Genetics and Molecular Oncology expanded the range of medical services provided in the field of diagnostic testing of hereditary (germinal) changes. In this regard, ZGiOM cooperates with the Oncological Genetics Outpatient Clinic (Polyclinic of the Oncology Centre) and the Department of Prevention and Health Promotion – Genetic Outpatient Clinic under the Ministry of Health’s program “Care for families with high, hereditary risk of developing malignant neoplasms – early detection of malignant neoplasms in families with high hereditarily conditioned risk of breast and ovarian cancer for the years 2019 – 2021”.
We perform both the assessment of the most common mutations in BRCA1/2, PALB2, CHEK2 using the PCR, qPCR and capillary sequencing techniques, as well as the assessment of known and unknown lesions using the NGS method (peripheral blood).
COOPERATION WITH RESEARCH AND SCIENCE CENTERS
The Department of Genetics and Molecular Oncology cooperates with numerous research and development centres both in Poland and abroad. The most significant of them are:
- Northwestern University in Chicago – cooperation in scientific research in the field of basic sciences, in order to identify mechanisms regulating gene expression related to the PNC structure. Many years of cooperation with Northwestern University resulted in obtaining by the Head of ZGiOM, Professor Marzena Lewandowska, MD, PhD, two research grants financed through national and international competitions:
- Department of Molecular Genetics, Polish Academy of Sciences, Poznań.
- Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Toruń.
QUALITY OF GENETIC TESTING AT THE HIGHEST LEVEL
In order to ensure high-quality of molecular tests performed at IFM – Department of Genetics and Molecular Oncology, we use CE-IVD certified reagents in the diagnostic process. In addition, the high quality of tests performed in our Department can be confirmed by the fact that we participate in numerous national and international quality controls. A positive assessment is confirmed each time by the awarded certificates.
We have been the leader among European laboratories continuously since 2012!
Certifications we participate in include: EMQN (United Kingdom), NEQAS (United Kingdom), GenQA (United Kingdom), EQA (Belgium).
Since 2012, we have had international certificates for performing genetic tests in neoplastic tissue using molecular techniques (qPCR) as well as in the field of molecular cytogenetics (FISH). A novelty (from 2018) are certificates for performing genetic tests using Next Generation Sequencing (NGS – ovarian cancer) or liquid biopsy – where the material for genetic testing is circulating cancerous DNA.
Lung cancer
Since 2011, we have been regularly taking part in international quality assessments of somatic mutation tests in the EGFR gene and ALK gene rearrangements, and for two years also in the ROS1 gene rearrangement in lung cancer. In addition, we took part in a pilot for European countries, assessment of the quality of ctEGFR mutation tests in the circulating cancerous DNA cfDNA EQA scheme 2018 – IQNPath. We have a current EMQN Lung Cancer validation certificate (NSCLC; EGFR, ALK, ROS1) issued in 2019 and 2020 for the maximum number of points (2/2): 100% genotyping, 100% interpretation, 100% formal correctness of the result. The average results obtained by 401 laboratories from around the World were respectively: 1.87/2.0, 1.61/2.0, 1.85/2.0). It is also noteworthy, that the assessors from the international commission verifying the assessment of mutations in the EGFR gene, ALK gene rearrangement and ROS1 gene rearrangement, beyond the scoring – additionally congratulated on the very good report on the genetic test.

Colon cancer
In 2018, the Department of Genetics and Molecular Oncology of the Oncology Centre in Bydgoszcz received also the EQA (External Quality Assessment) certification: Colon. The quality control concerned the performance and molecular analysis of somatic mutations in three genes: KRAS, NRAS and BRAF. 112 laboratories from 27 countries participated in it, including 7 laboratories from Poland. As many as 15% of laboratories were not certified at all. Our Department obtained the maximum score for correct genotyping (20/20). It is also a success that we received a full score (4/4) for reporting, i.e. correctly presenting the results along with the interpretation.
Ovarian cancer – NGS method
Since 2018, it is possible to undergo international quality control of performance of germinal and somatic mutation tests in BRCA1 and BRCA2 genes using the NGS method, organized by EMQN in the United Kingdom. In 2018, we obtained the maximum number of points, placing ourselves in the forefront of European laboratories. The external evaluation of EMQN BRCA1/2 2018 was a pilot event because laboratories in European countries, like our Department, have been preparing for the implementation of this service in recent years. The validation assessed: genotyping, result interpretation and the formal correctness of the result, and the average results from 220 laboratories from all over Europe were respectively: 1.85/2; 1.62/2 and 1.96/2 points. Our laboratory was the only one in the Kuyavian-Pomeranian Voivodeship that participated in this assessment.
Breast cancer
We are certified to perform tests in the field of molecular cytogenetics (simple genetic test – evaluation of HER2 gene amplification using the FISH method) in breast cancer. In 2017, we took part in a 3-stage external laboratory validation organized by The United Kingdom National External Quality Assessment Services (UK NEQAS). The “Excellent Standard” result we received distinguishes us from other laboratories around the World. The points awarded to us by the UK NEQAS placed the Department of Genetics and Molecular Oncology in a very small select group of laboratories providing services at an excellent level. It should be noted that 20% of the laboratories that undergo the HER2 gene amplification assessments and reported for quality assessment – did not pass the qualification. This result shows that although the technique and evaluation appear to be a simple method, the experience in genetic testing, high competence and adherence to the highest standards in routine diagnostics allowed us to pass the international evaluation of the quality of HER2 amplification with very good results.
Melanoma
In the international evaluation of molecular analyses of somatic mutations in the BRAF gene (UK NEQAS for Molecular Genetics) in 2017, the Department of Genetics and Molecular Oncology achieved an outstanding result – the maximum number of points, once again confirming the highest quality of our services performed using real-time PCR.
Achieving such high results in International Quality Assessments every year is a guarantee for patients of the Oncology Centre that genetic tests will be performed at the highest level. This is of particular significance because these tests are the basis for qualifying patients for molecularly targeted therapies.
In accordance with the guidelines of the Minister of Health (developed by the Team for the coordination of the network of COVID laboratories – update on December 31, 2020) and in response to the appeal of the Marshal of the Kujawsko-Pomorskie Voivodeship, Mr. Piotr Całbecki, concerning the combining of efforts and reporting in of units that can support our voivodeship in the fight against the third epidemic wave, as on April 2, 2021, the Department of Genetics and Molecular Oncology was entered into the list of COVID laboratories.

|
Kierownik
prof. dr hab. n. med. Marzena Lewandowska
|

