Department of Nuclear Medicine

PET/CT
PET/CT is a combination of two diagnostic tests: positron emission tomography (PET) and computed tomography (CT). The PET method allows to visualize the functions of tissues and organs. It works based on an analysis of the accumulation of markers (e.g. glucose) in the tissues. Glucose is most needed by metabolically active tissues, which voracious cells absorb it in enormous amounts, such a tissue is the cancerous one.
Computed Tomography, on the other hand, consists in x-rays of a given area of the body and measuring their absorption by tissues. In this way, the doctor obtains a cross-section of a given part of the body.
In the PET/CT method, PET shows that there is an area in the body that shows metabolic activity, i.e. a probable disease focus, but does not give a specific location. Working together with Computed Tomography which allows to precisely define them.
Our centre, as one of few in the country, can independently produce markers for the needs of PET/CT and PET/MR diagnostic tests.
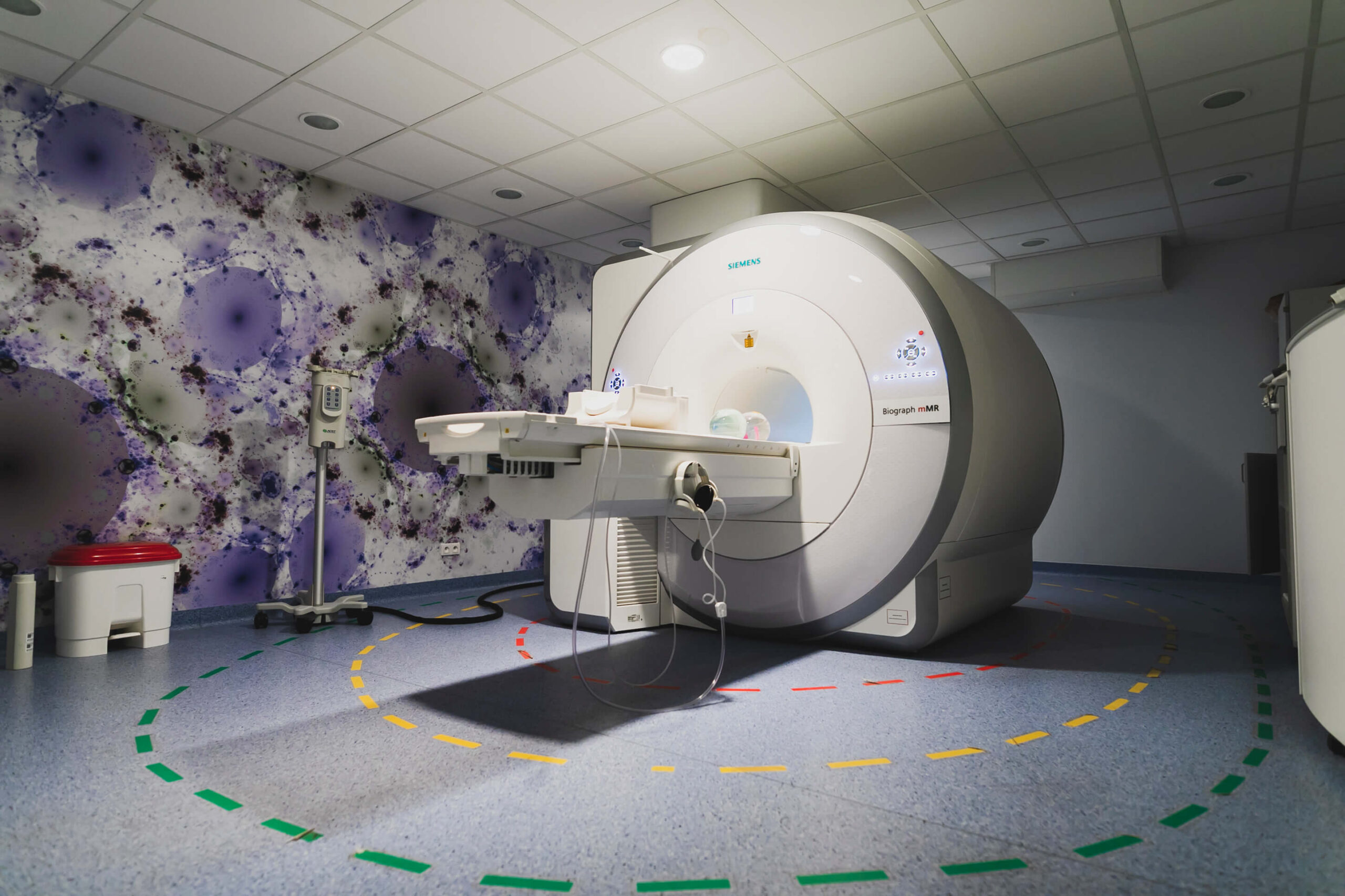
PET/MR
The Oncology Centre in Bydgoszcz is the first hospital in Poland to have PET/MR technology.
Magnetic resonance imaging (MR) is one of the most accurate forms of diagnosis. It works well in detecting neoplasms, metastases, lesions in tissues.
The MR technique uses the properties of hydrogen protons (a component of water in our body), which become magnetized in a strong EM field and begin to send impulses. These impulses are processed by electronic devices, thus creating an image of the examined tissues, visible on the monitor.
The test is performed in one procedure because the scanners are connected and the data is downloaded at the same time.
Similarly as with PET/CT, one device is responsible for showing the anatomy (MR), the other is for the physiological function (PET). MR has an excellent contrast of soft tissues. PET/MR has a very high image resolution and the examination does not use ionizing radiation.
PET/MR examination is not included in the pack of services guaranteed by the National Health Fund (NFZ). Qualification for the examination takes place at the Department of Nuclear Medicine by appointment.
How to register?
PET/CT and PET/MR examinations are performed at the Department of Nuclear Medicine in building C. In order to register for a PET/CT examination, please send a referral to the Department’s secretariat office, phone number: 52 374 34 28 and provide your current telephone number. After verification of the referral, a person responsible for registration contacts the patient.
Feel free to contact us via e-mail at the address: sekretariatzmn@co.bydgoszcz.pl
PET scanning is used to:
- diagnose early neoplastic conditions,
- check if cancer has metastasized to other organs,
- accurately locate pathological lesions,
- monitor the effects of the therapy,
- look for signs of inflammation throughout the entire body.
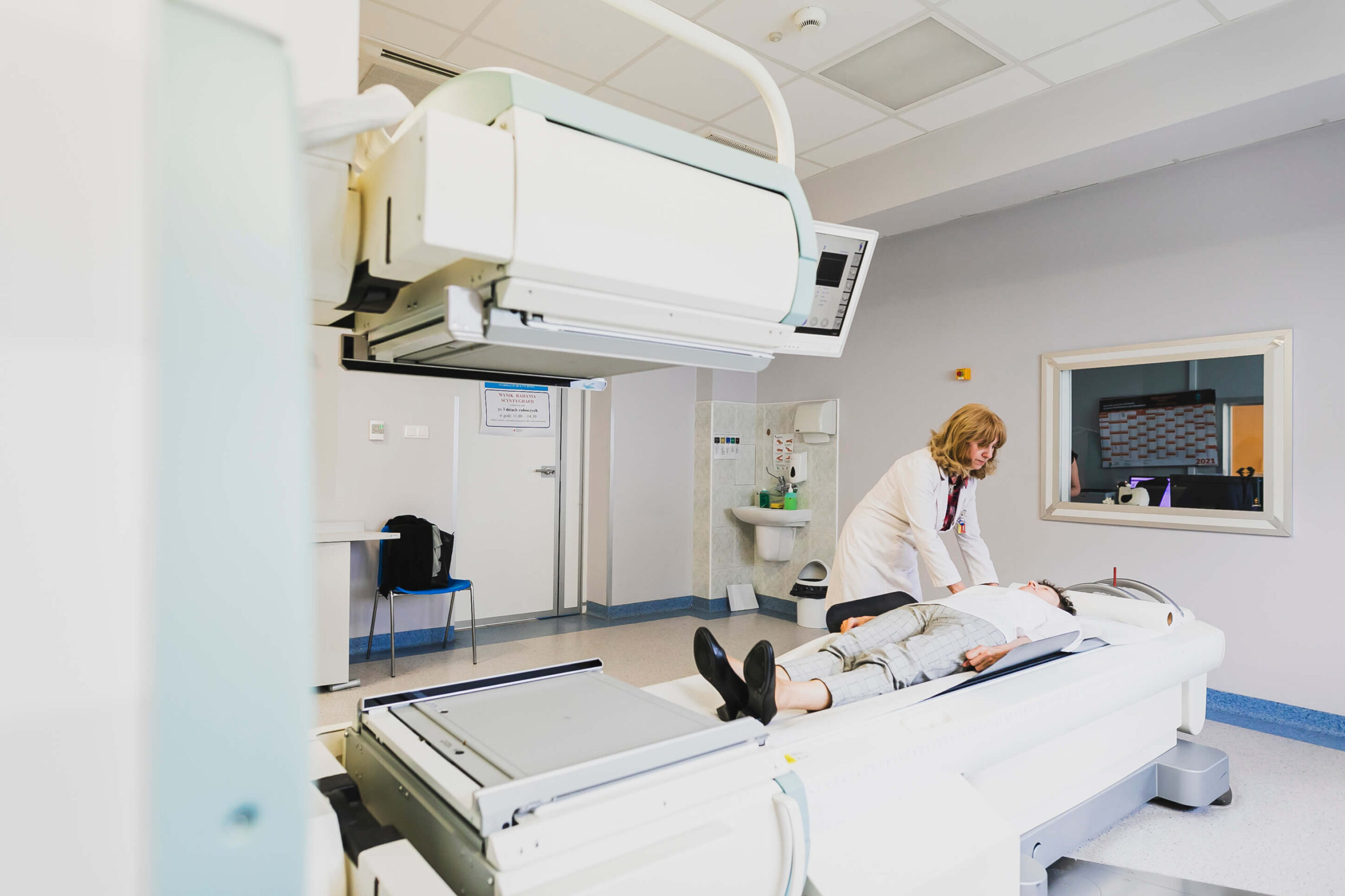
SCYNTIGRAPHY
Scintigraphy is an imaging examination in which the human internal organs are visualized using radioactive isotopes. It is performed mainly in the case of neoplastic disease. It is completely safe, and the isotopes are quickly excreted from the body.
Scintigraphic studies use small doses of radiation-emitting isotopes, usually combined with chemical compounds that cause their accumulation in a specific organ. The isotopes in the body are imaged using a scintigraphy or a gamma camera. A digital image of the distribution of isotope accumulation in the body is captured on the computer.
Scintigraphy is performed in order to obtain a graphical overview of the organs, primarily to assess their position, size, structure and flow. Thanks to this method, it is possible to visualize the circulation of haemoglobin, urine and bile.
Indications for scintigraphy
- cancers
- suspicion of neoplastic metastases
- gastrointestinal bleeding
- suspected kidney disease
- suspected abnormalities in the circulation of the cerebrospinal fluid
- repeated pneumonia
- diseases of the heart muscle
- chronic hepatitis

Radiopharmaceuticals Production Laboratory
At the Oncology Centre of F. Łukaszczyk in Bydgoszcz, within the structure of the Department of Nuclear Medicine functions a modern Radiopharmaceuticals Production Laboratory, comprising also the Radiopharmaceuticals Factory of the Oncology Centre.
Thanks to the radioactive properties of radiopharmaceuticals used in imaging diagnostics, we are able to locate neoplastic foci in their very early stages of development, and thus combat the disease more effectively and efficiently.
The activity of the Radiopharmaceuticals Production Laboratory includes labelling scintigraphic diagnostic kits with technetium Tc99m and gallium Ga68, as well as dispensing ready-made radiopharmaceuticals for therapy with iodine I131, samarium Sm153, radium Ra223 and yttrium Y90.
The Radiopharmaceuticals Factory has modern production and quality control laboratories and a cyclotron laboratory, as well as a highly specialized pharmaceutical microbiology laboratory located at the Department of Microbiology.
As a result of a positively completed inspection carried out by the inspectors of the Main Pharmaceutical Inspectorate, the Radiopharmaceuticals Factory at the Oncology Centre in Bydgoszcz obtained, by Decision no. GIF-IW-400/0463/01/484/31/15, a permit to manufacture or import the medicinal product FDG POZYTON (active substance: Fluodeoxyglucose [18F]).
On May 26, 2021, the Radiopharmaceuticals Factory of the Oncology Centre in Bydgoszcz, obtained, by decision of the President of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products, license no. 26425 for marketing the medicinal product FDG POZYTON.
The Radiopharmaceuticals Factory of the Oncology Centre in Bydgoszcz offers the medicinal product FDG POZYTON 250-3100 MBq/mL for the duration of calibration – a solution for injection, manufactured in supervised conditions that meet the requirements of Good Manufacturing Practice, thus ensuring the highest quality product. The high standard of production is also achieved with the participation of qualified staff, whose extensive experience is confirmed by many years of production of radiopharmaceuticals for the Oncology Centre in Bydgoszcz. The entire product manufacturing process is documented in detail.
Our main objective in line with the implemented and maintained Pharmaceutical Quality System and the approved Quality Policy
is to maintain the high standard of the Factory’s operations with the simultaneous striving for continuous improvement of the processes, including, above all, providing customers with the highest quality product.


REPORTING OF ADVERSE SIDE EFFECTS OF A MEDICINAL PRODUCT
The Radiopharmaceuticals Factory of the Oncology Centre in Bydgoszcz registers and monitors all reports of adverse side effects of the FDG POZYTON medicinal product.
If you observe any adverse side effects during the use of FDG POZYTON 250-3100 MBq/ml – solution for injection, please inform us immediately about their occurrence.
Summary of Medicinal Product Characteristics /available on the website of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products at www.urpl.gov.pl/
An adverse side effect of a medicinal product is any unfavourable and unintended effect of a medicinal product.
Please report adverse side effects:
1. by phone at the following numbers:
Person Responsible for Pharmacotherapy Safety Supervision, phone number: 790 500 725
Manufacturing Coordinator at the Radiopharmaceuticals Factory, phone number: 52 374 37 50
2. e-mail: chudyp@co.bydgoszcz.pl
3. Fax number: 52 374 33 01

|
Coordinator of the Department of Nuclear Medicine
Bogdan Małkowski, MD, PhD
|
|
Coordinator at the Radiopharmaceuticals Production Laboratory
Jolanta Czuczejko, MD, PhD
|

